
Chemistry, 18.03.2021 21:20 vanitycarraway2000
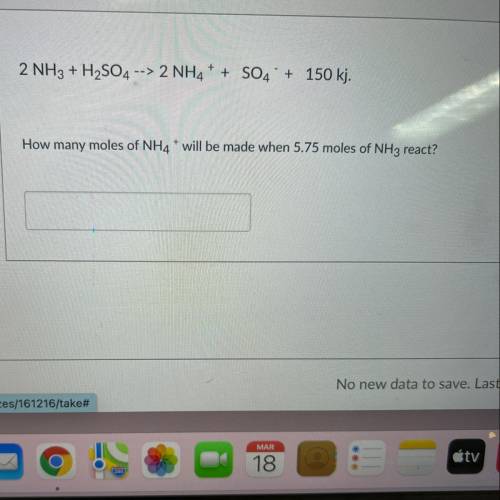
2 NH3 + H2SO4 --> 2NH4+ + SO4 + 150 kj. How many moles of NH4* will be made when 5.75 moles of NH3 react?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1


You know the right answer?
2 NH3 + H2SO4 --> 2NH4+ + SO4 + 150 kj.
How many moles of NH4* will be made when 5.75 moles of N...
Questions

Mathematics, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40


Chemistry, 21.01.2021 02:40



Mathematics, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40


Mathematics, 21.01.2021 02:40

English, 21.01.2021 02:40




Chemistry, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40

Mathematics, 21.01.2021 02:40




