
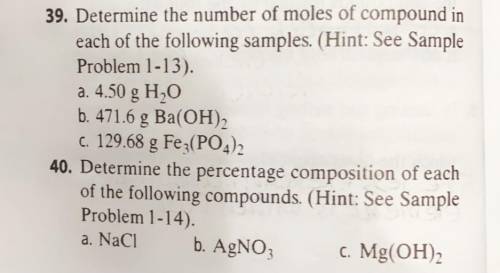
39. Determine the number of moles of compound in
each of the following samples. (Hint: See Sample
Problem 1-13).
a. 4.50 g H2O
b. 471.6 g Ba(OH)2
C. 129.68 g Fe3(PO4)2
40. Determine the percentage composition of each
of the following compounds. (Hint: See Sample
Problem 1-14).
b. AgNO3
.c. Mg(OH)2
a. NaCl
Help me please for this two question

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
39. Determine the number of moles of compound in
each of the following samples. (Hint: See Sample
Questions




History, 26.07.2019 21:00


History, 26.07.2019 21:00


English, 26.07.2019 21:00

English, 26.07.2019 21:00













