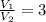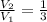Chemistry, 18.03.2021 14:00 nelsoneligwe7
1. The pressure of a gas is 100.0 kPa and its volume is 500.0 ml. If the volume increases to 1,000.0 ml, what is the new pressure of the gas?
2. If a gas at 25.0 °C occupies 3.60 liters at a pressure of 10 kPa, what will be its volume at a pressure of 25 kPa?
3. When the pressure on a gas increases three times, by how much will the volume increase or decrease?
4. Boyle's Law deals what quantities?

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Scientist wants to react a barium chloride salt with an acid. to speed up the reaction, the scientist crushes the barium chloride salt until it is a fine powder. this speeds up the reaction by a. increasing the ph of the salt. b. increasing the surface area of the salt. c. increasing the pressure of the salt. d. increasing the temperature of the salt. im thinking more a not 100% sure
Answers: 3

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1


Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
1. The pressure of a gas is 100.0 kPa and its volume is 500.0 ml. If the volume increases to 1,000.0...
Questions

Geography, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01





Mathematics, 16.10.2020 22:01


Mathematics, 16.10.2020 22:01



Chemistry, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01

History, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01


Geography, 16.10.2020 22:01

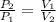 (1)
(1)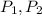 - Initial and final pressure, measured in kPa.
- Initial and final pressure, measured in kPa.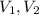 - Initial and final pressure, measured in mililiters.
- Initial and final pressure, measured in mililiters.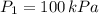 ,
, 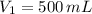 and
and 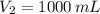 , then the new pressure of the gas is:
, then the new pressure of the gas is: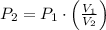
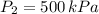
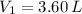 ,
, 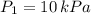 and
and 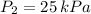 then the new volume of the gas is:
then the new volume of the gas is: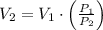
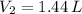
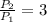 , then the volume ratio is:
, then the volume ratio is: