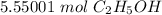Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
How many moles of ethanol are produced starting with 500.g glucose?
C6H12O6 → 2 C2H5OH + 2 CO2...
Questions

Chemistry, 26.01.2021 03:40

Chemistry, 26.01.2021 03:40


Mathematics, 26.01.2021 03:40



History, 26.01.2021 03:40



English, 26.01.2021 03:40

Chemistry, 26.01.2021 03:40

English, 26.01.2021 03:40

Health, 26.01.2021 03:40






Mathematics, 26.01.2021 03:40

Mathematics, 26.01.2021 03:40

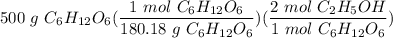 [DA} Multiply/Divide [Cancel out units]:
[DA} Multiply/Divide [Cancel out units]: