
Chemistry, 18.03.2021 09:00 jazzy76783
6HCl(aq) + 2A1)
-
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1.20 moles of aluminum?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
6HCl(aq) + 2A1)
-
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1...
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1...
Questions

Biology, 28.05.2020 19:04

Chemistry, 28.05.2020 19:04

Mathematics, 28.05.2020 19:04

Health, 28.05.2020 19:04

Mathematics, 28.05.2020 19:04

History, 28.05.2020 19:04



Mathematics, 28.05.2020 19:04

Mathematics, 28.05.2020 19:04



Mathematics, 28.05.2020 19:04

Mathematics, 28.05.2020 19:04


Mathematics, 28.05.2020 19:04


English, 28.05.2020 19:04

Mathematics, 28.05.2020 19:04

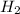 will be produced 1.20 moles of aluminium.
will be produced 1.20 moles of aluminium.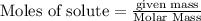
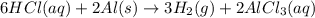
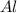 produce = 3 moles of
produce = 3 moles of 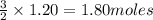 of
of 

