
Chemistry, 18.03.2021 09:10 xelynncaldera
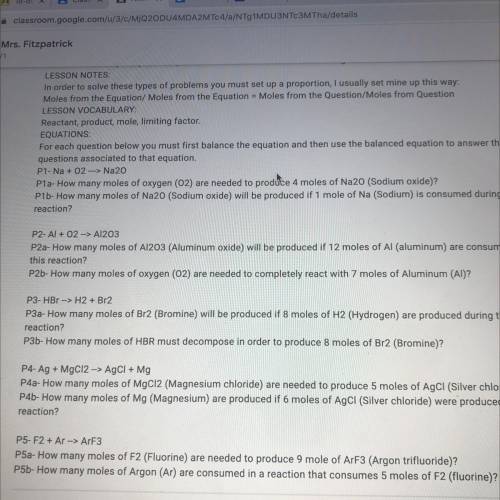
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (Argon trifluoride)?
P5b- How many moles of Argon (Ar) are consumed in a reaction that consumes 5 moles of F2 (fluorine)?
I need the answers for the p5 part please help

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (A...
Questions

Advanced Placement (AP), 22.01.2021 01:00

Advanced Placement (AP), 22.01.2021 01:00

Mathematics, 22.01.2021 01:00


Mathematics, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00




English, 22.01.2021 01:00



Social Studies, 22.01.2021 01:00


Arts, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00


Biology, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00



