
Chemistry, 18.03.2021 08:50 mrylenastewart
How many moles of CO2 are produced
when 10.0 moles of C3H8 are burned in
excess oxygen?
C3H8 + 5 O2 ---> 3 CO2 + 4H2O

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
How many moles of CO2 are produced
when 10.0 moles of C3H8 are burned in
excess oxygen?
...
excess oxygen?
...
Questions

History, 18.04.2020 18:47

Social Studies, 18.04.2020 18:47


History, 18.04.2020 18:48

Mathematics, 18.04.2020 18:48




English, 18.04.2020 18:48

Social Studies, 18.04.2020 18:48


Biology, 18.04.2020 18:48




Mathematics, 18.04.2020 18:48


English, 18.04.2020 18:48

Physics, 18.04.2020 18:48

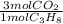 = 30.0 mol CO₂
= 30.0 mol CO₂

