
Chemistry, 18.03.2021 07:20 makaylagrandsta
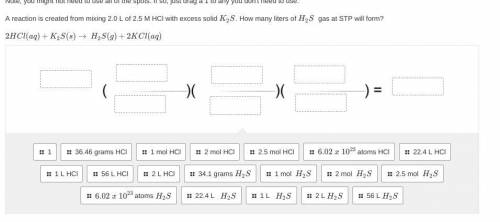
Please only answer w help and i will give you brainliest. <3 A reaction is created from mixing 2.0 L of 2.5 M HCl with excess solid K2S. How many liters of H2S gas at STP will form?
2HCl(aq)+K2S(s)→ H2S(g)+2KCl(aq)
(picture below)
-
C3H8+5O2 → 3 CO2 +4H2O
If 33.6 liters of O2 gas at STP react with 55 grams of C3H8, what is the limiting reactant? How much water can be formed (in grams)?
thank you!!!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
Please only answer w help and i will give you brainliest. <3 A reaction is created from mixing 2....
Questions

Mathematics, 03.12.2019 17:31

History, 03.12.2019 17:31


Mathematics, 03.12.2019 17:31

Mathematics, 03.12.2019 17:31


Mathematics, 03.12.2019 17:31


Mathematics, 03.12.2019 17:31


English, 03.12.2019 17:31

Social Studies, 03.12.2019 17:31

English, 03.12.2019 17:31

Mathematics, 03.12.2019 17:31

English, 03.12.2019 17:31

Mathematics, 03.12.2019 17:31

Geography, 03.12.2019 17:31

Mathematics, 03.12.2019 17:31

Biology, 03.12.2019 17:31




