How does Nitrogen Dioxide
react with :
I. Copper
ii. Phosphorus.
iii. Sodium h...

Chemistry, 18.03.2021 04:40 BreBreDoeCCx
How does Nitrogen Dioxide
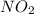
react with :
I. Copper
ii. Phosphorus.
iii. Sodium hydroxide.
Note you are asked to explain the reaction that occurs between Nitrogen Dioxide and each of the given elements with the aid of a chemical equation.
State what type of reaction that occurs.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
Questions










Physics, 24.01.2020 00:31






English, 24.01.2020 00:31

Spanish, 24.01.2020 00:31





