Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
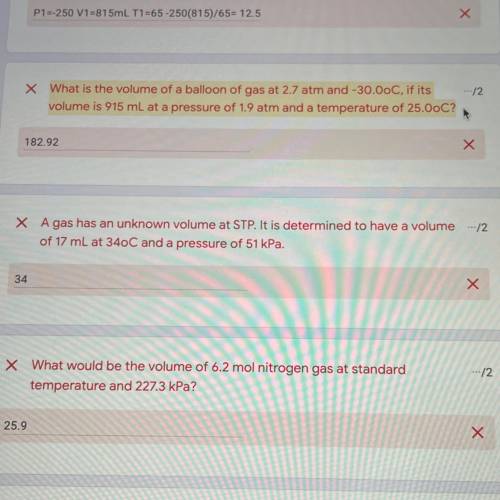
What is the volume of balloon of gas at 2.7 atm and -30.0oC if it’s volume is 915mL at pressure of 1...
Questions

Biology, 25.09.2019 04:00


Advanced Placement (AP), 25.09.2019 04:10



Chemistry, 25.09.2019 04:10

Biology, 25.09.2019 04:10

Mathematics, 25.09.2019 04:10


Chemistry, 25.09.2019 04:10


English, 25.09.2019 04:10



Mathematics, 25.09.2019 04:10