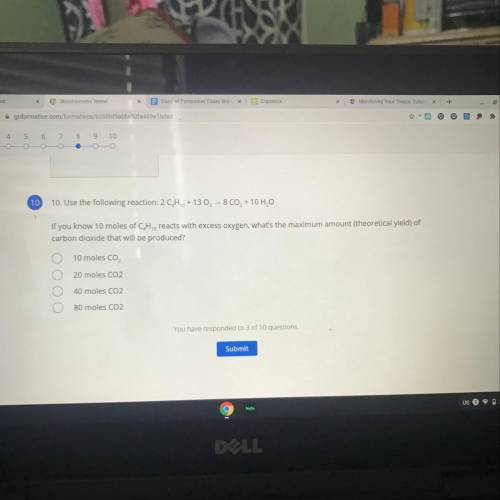
Use the following reaction:
2 CH2 + 13 O2 - 8 CO2 + 10 H2O
If you know 10 moles of C, H, reac...

Chemistry, 18.03.2021 03:30 anonymousanon
Use the following reaction:
2 CH2 + 13 O2 - 8 CO2 + 10 H2O
If you know 10 moles of C, H, reacts with excess oxygen, what's the maximum amount (theoretical yield) of
carbon dioxide that will be produced?
A. 10 moles CO,
B. 20 moles CO2
C. 40 moles CO2
D. 80 moles CO2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Questions

Mathematics, 23.05.2021 23:20


English, 23.05.2021 23:20





Physics, 23.05.2021 23:20


Social Studies, 23.05.2021 23:20


Computers and Technology, 23.05.2021 23:20

Mathematics, 23.05.2021 23:20

English, 23.05.2021 23:20

Mathematics, 23.05.2021 23:20



Chemistry, 23.05.2021 23:20

Mathematics, 23.05.2021 23:20

Business, 23.05.2021 23:20



