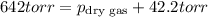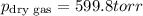Chemistry, 18.03.2021 03:10 superfly903
A sample of gas is collected over water at a temperature of 35.0◦C when the barometric pressure reading is 642 torr. What is the partial pressure of the dry gas, given PH2O = 42.2 torr? Answer in units of torr.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

You know the right answer?
A sample of gas is collected over water at a temperature of 35.0◦C when the barometric pressure read...
Questions



Physics, 22.07.2019 06:00

Business, 22.07.2019 06:00

Mathematics, 22.07.2019 06:00




Chemistry, 22.07.2019 06:00


English, 22.07.2019 06:00



Chemistry, 22.07.2019 06:00



Social Studies, 22.07.2019 06:00



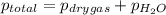
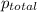 =total pressure of gases = 642 torr
=total pressure of gases = 642 torr
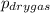 = partial pressure of dry gas = ?
= partial pressure of dry gas = ?
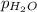 = partial pressure of water = 42.2 torr
= partial pressure of water = 42.2 torr