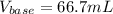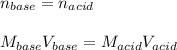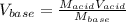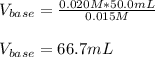Chemistry, 18.03.2021 02:50 ldibut2003
For the titration of 50.0 mL of .020M HI with 0.015 M of NaOH, graph pH versus milliliters of base added from 0-100 mL. How many milliliters of NaOH are added at the equivalence point?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
For the titration of 50.0 mL of .020M HI with 0.015 M of NaOH, graph pH versus milliliters of base a...
Questions

Biology, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00









Biology, 02.02.2021 01:00


Mathematics, 02.02.2021 01:00




Mathematics, 02.02.2021 01:00



Biology, 02.02.2021 01:00