
Chemistry, 18.03.2021 02:40 HalpMahOnMahH0meW0rk
the reaction of sodium peroxide (Na2O2) and water produces sodium hydroxide and oxygen gas. the following chemical equation represents the reaction. balance the equation before beginning the problems. ( use the equation for problems one and two )
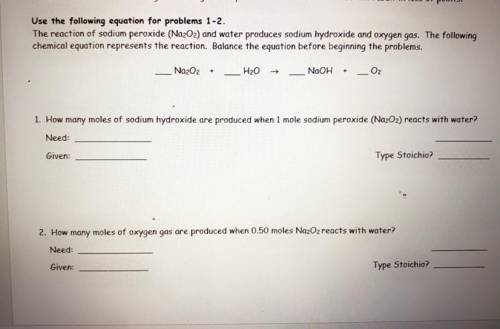

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2
You know the right answer?
the reaction of sodium peroxide (Na2O2) and water produces sodium hydroxide and oxygen gas. the foll...
Questions

Social Studies, 19.07.2019 13:30

Biology, 19.07.2019 13:30

Mathematics, 19.07.2019 13:30

Social Studies, 19.07.2019 13:30

Social Studies, 19.07.2019 13:30

Social Studies, 19.07.2019 13:30

Mathematics, 19.07.2019 13:30

Social Studies, 19.07.2019 13:30

History, 19.07.2019 13:30

English, 19.07.2019 13:30

Biology, 19.07.2019 13:30

History, 19.07.2019 13:30

Mathematics, 19.07.2019 13:30

Mathematics, 19.07.2019 13:30




Mathematics, 19.07.2019 13:30

French, 19.07.2019 13:30



