Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
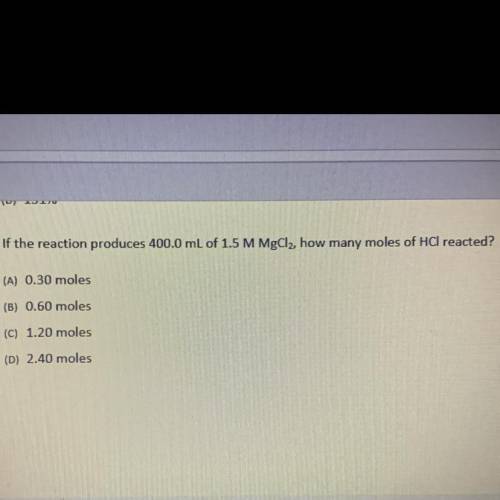
If the reaction produces 400.0 mL of 1.5 M MgCl2, how many moles of HCl reacted?
(A) 0.30 moles
Questions

Mathematics, 29.01.2020 07:04





Business, 29.01.2020 07:04

Mathematics, 29.01.2020 07:04

History, 29.01.2020 07:04

Mathematics, 29.01.2020 07:04





Social Studies, 29.01.2020 07:04




History, 29.01.2020 07:04

Mathematics, 29.01.2020 07:04