
Chemistry, 18.03.2021 02:10 krystlemiller11211
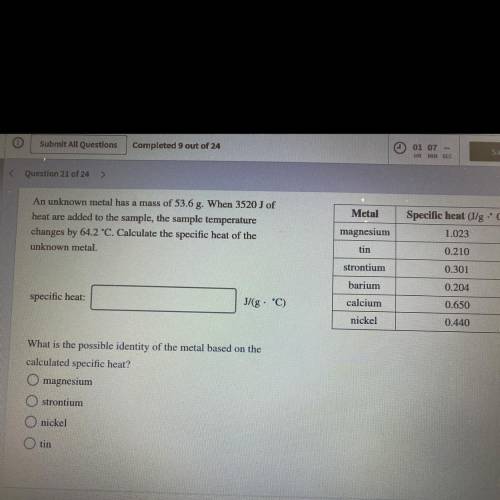
An unknown metal has a mass of 53.6 g. When 3520J of heat are added to the sample, the sample temp. changes by 64.2°C. Calculate specific heat of the unknown metal. what is the metal?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
An unknown metal has a mass of 53.6 g. When 3520J of heat are added to the sample, the sample temp....
Questions

Social Studies, 15.07.2019 05:50

Mathematics, 15.07.2019 05:50

Biology, 15.07.2019 05:50

Social Studies, 15.07.2019 05:50


Chemistry, 15.07.2019 05:50

Biology, 15.07.2019 05:50

Social Studies, 15.07.2019 05:50

Biology, 15.07.2019 05:50

Mathematics, 15.07.2019 05:50

History, 15.07.2019 05:50


History, 15.07.2019 05:50


Social Studies, 15.07.2019 05:50



Social Studies, 15.07.2019 05:50

Biology, 15.07.2019 05:50



