
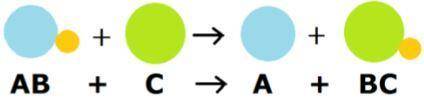
Looking at the image, what does it mean?
I learned: Enthalpy values, Entropy, Calorimetry, Reaction Rates, Equilibrium, and Principle.
My first thought was it is a single replacement reaction, but I feel like there is more to it.
Please no false answers. Thanks much & have a good day!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Looking at the image, what does it mean?
I learned: Enthalpy values, Entropy, Calorimetry, Reaction...
Questions

Business, 09.02.2021 15:50

Social Studies, 09.02.2021 15:50

German, 09.02.2021 15:50


Mathematics, 09.02.2021 15:50

Biology, 09.02.2021 15:50




Mathematics, 09.02.2021 15:50

Mathematics, 09.02.2021 15:50

Mathematics, 09.02.2021 15:50

Mathematics, 09.02.2021 15:50

English, 09.02.2021 15:50

Mathematics, 09.02.2021 15:50







