Determining Reaction Rate
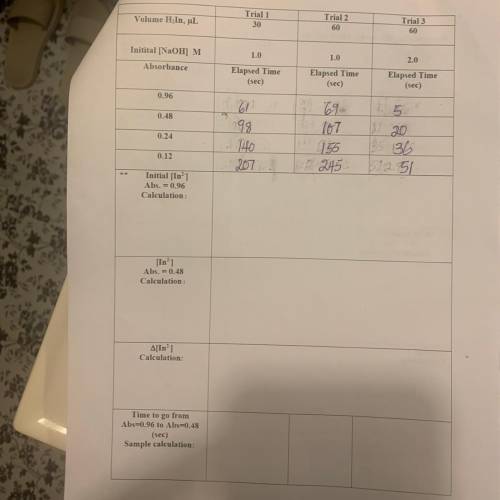
Volume H, In, uL
Trial 1
30
Trial 2
60
Tria...

Chemistry, 18.03.2021 02:10 fdryktysysmzet3640
Determining Reaction Rate
Volume H, In, uL
Trial 1
30
Trial 2
60
Trial 3
60
1.0
1.0
2.0
Initital (NaOH) M
Absorbance
Elapsed
Time
(sec)
Elapsed Time
(sec)
Elapsed Time
(sec)
0.96
0.48
61
198
140
0.24
107
155
245
20
36
0.12
Initial [Inº]
Abs. = 0.96
Calculation :
Abs. = 0.48
Calculation :
A[Inº]
Calculation:
x
Time to go from
Abs=0.96 to Abs=0.48
(sec)
Sample calculation:

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Questions



English, 26.10.2021 02:10

Mathematics, 26.10.2021 02:10


Social Studies, 26.10.2021 02:10

Mathematics, 26.10.2021 02:10



Mathematics, 26.10.2021 02:10

Mathematics, 26.10.2021 02:10


English, 26.10.2021 02:10


English, 26.10.2021 02:10

Mathematics, 26.10.2021 02:10


Mathematics, 26.10.2021 02:10


Mathematics, 26.10.2021 02:10



