1. What is the wavelength (in meters) of a radio wave with a frequency of 9.31 x Hz?
2....

Chemistry, 18.03.2021 02:00 nathaniel12
1. What is the wavelength (in meters) of a radio wave with a frequency of 9.31 x 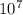 Hz?
Hz?
2. Calculate the energy of:
a) a photon with a wavelength of 5.00 x 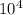 nm.
nm.
b) a photon with a wavelength of 5.00 x 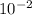 nm.
nm.
3. The energy of a photon is 5.87 x 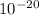 J. What is its wavelength in nm?
J. What is its wavelength in nm?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1


Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Questions

Mathematics, 12.11.2019 07:31




Chemistry, 12.11.2019 07:31



Mathematics, 12.11.2019 07:31



Mathematics, 12.11.2019 07:31






French, 12.11.2019 07:31

Mathematics, 12.11.2019 07:31


Social Studies, 12.11.2019 07:31




