
Chemistry, 18.03.2021 01:30 tristantisdale1
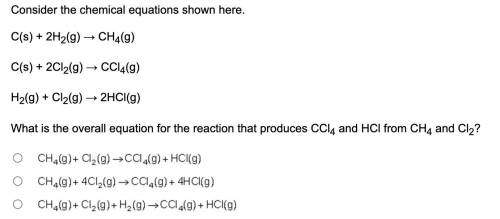
Consider the chemical equations shown here. C(s) + 2H2(g) → CH4(g) C(s) + 2Cl2(g) → CCl4(g) H2(g) + Cl2(g) → 2HCl(g) What is the overall equation for the reaction that produces CCl4 and HCl from CH4 and Cl2? Upper C upper H subscript 4 (g) plus upper C l subscript 2 (g) right arrow upper C upper C l subscript 4 (g) plus upper H upper C l (g). Upper C upper H subscript 4 (g) plus 4 upper C l subscript 2 (g) right arrow upper C upper C l subscript 4 (g) plus 4 upper H upper C l (g). Upper C upper H subscript 4 (g) plus upper C l subscript 2 (g) plus upper H 2 (g) right arrow upper C upper C l subscript 4 (g) plus upper H upper C l (g).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1

Chemistry, 23.06.2019 14:00
Which word refers to the smallest functional unit of living thing
Answers: 1
You know the right answer?
Consider the chemical equations shown here. C(s) + 2H2(g) → CH4(g) C(s) + 2Cl2(g) → CCl4(g) H2(g) +...
Questions

Mathematics, 15.04.2021 23:40





Mathematics, 15.04.2021 23:40

Mathematics, 15.04.2021 23:40










Mathematics, 15.04.2021 23:40





