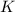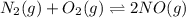Chemistry, 18.03.2021 01:10 alexandrecarmene
The equilibrium-constant expression is used to describe the concentration of reactants and products for a reaction in dynamic equilibrium. For ideal gases and ideal solutions in homogeneous equilibria, where all reactants and products are in the same phase, the extent to which a particular chemical reaction proceeds to products is given by the equilibrium equation.
aA+bB⇌cC+dD , K=[C]c[D]d[A]a[B]b
where K is the equilibrium constant and the right-hand side of the equation is known as the equilibrium-constant expression.
The concentration of each product raised to its coefficient is divided by the concentration of each reagent raised to its coefficient according the the balanced chemical equation. Therefore, the higher the concentration of products, the larger the value of K will be.
Required:
Identify the proper form of the equilibrium-constant expression for the equation.
N2(g)+O2(g)⇌2NO(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
The equilibrium-constant expression is used to describe the concentration of reactants and products...
Questions


Mathematics, 21.06.2019 15:30

Computers and Technology, 21.06.2019 15:30









English, 21.06.2019 15:30






Biology, 21.06.2019 15:30


![K=\frac{[NO]^2}{[N_2]^1[O_2]^1}](/tpl/images/1195/8832/df6dc.png)