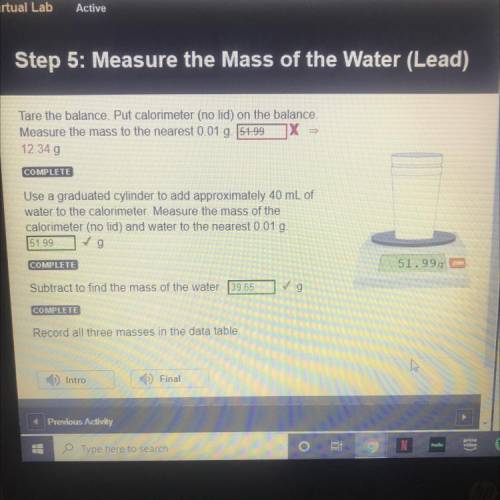
STEP 5: LEAD
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to t...

Chemistry, 17.03.2021 23:50 bartekpiglo
STEP 5: LEAD
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g.
12.34 g
Use a graduated cylinder to add approximately 40 mL of
water to the calorimeter. Measure the mass of the
calorimeter (no lid) and water to the nearest 0.01 g.
51.99 g
Subtract to find the mass of the water. 39.65 g

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
Questions

History, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00




Mathematics, 23.04.2021 01:00



Mathematics, 23.04.2021 01:00







History, 23.04.2021 01:00


Social Studies, 23.04.2021 01:00



