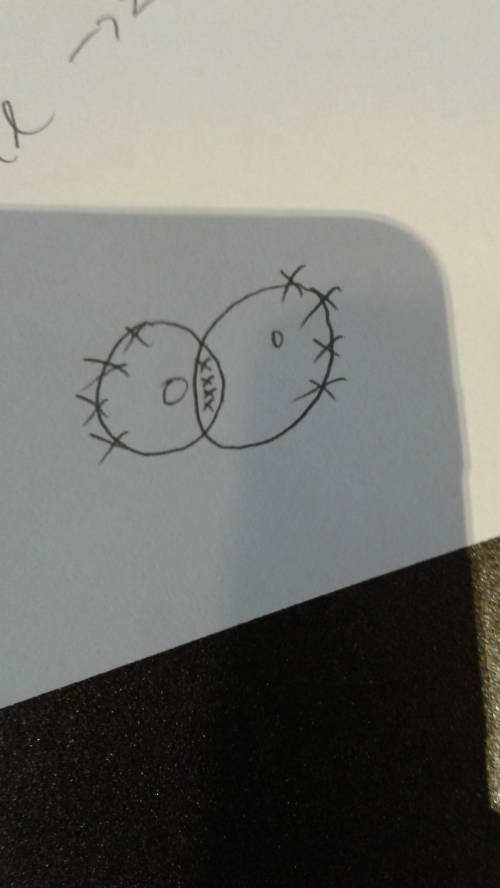
An oxygen atom has six electrons in its outermost energy level.
explain why two oxygen at...

Chemistry, 29.09.2019 18:30 BreBreDoeCCx
An oxygen atom has six electrons in its outermost energy level.
explain why two oxygen atoms must share four electrons when they form a covalent bond?
answer in complete sentences and thoroughly. 5-7

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Questions



Mathematics, 10.02.2021 04:00

Mathematics, 10.02.2021 04:00




Biology, 10.02.2021 04:00

Chemistry, 10.02.2021 04:00

Mathematics, 10.02.2021 04:00



Mathematics, 10.02.2021 04:00


Mathematics, 10.02.2021 04:00

Mathematics, 10.02.2021 04:00

Mathematics, 10.02.2021 04:00

Mathematics, 10.02.2021 04:00


Computers and Technology, 10.02.2021 04:00