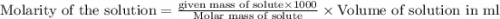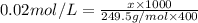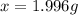Chemistry, 13.03.2021 14:00 tiffxnnyyy
Please if you know help
please
the formula of copper sulfate crystals is CuSo4.5H2O. 400ml of 0.02mol/l copper (ii) sulfate solution is needed to be prepered .
a. calculate the molar mass of the solute .
b. calculate the mass of the solute that sould be weighted to prepare this solution .
c. describe in details the steps of preperation of the solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1
You know the right answer?
Please if you know help
please
the formula of copper sulfate crystals is CuSo4.5H2O. 40...
the formula of copper sulfate crystals is CuSo4.5H2O. 40...
Questions

Mathematics, 18.11.2020 01:00


Health, 18.11.2020 01:00


Social Studies, 18.11.2020 01:00

Social Studies, 18.11.2020 01:00

Geography, 18.11.2020 01:00





Mathematics, 18.11.2020 01:00






Health, 18.11.2020 01:00


Arts, 18.11.2020 01:00

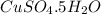 and dissolve in water until the volume is 400 ml
and dissolve in water until the volume is 400 ml