Hydrobromic acid dissolves solid iron according to the
following reaction:
Fe(s) + 2 HBr(aq)...

Chemistry, 13.03.2021 08:10 moranmar2283
Hydrobromic acid dissolves solid iron according to the
following reaction:
Fe(s) + 2 HBr(aq) → FeBr2(aq) + H2(9)
Part A
What mass of HBr (in g) would you need to dissolve a 3.2 - g pure iron
Express your answer using two significant figures.
Part B
What mass of H, would be produced by the complete reaction of the iron

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1
You know the right answer?
Questions

Mathematics, 12.12.2021 16:40



Chemistry, 12.12.2021 16:40

Social Studies, 12.12.2021 16:40

English, 12.12.2021 16:40

Mathematics, 12.12.2021 16:40

World Languages, 12.12.2021 16:40

English, 12.12.2021 16:40

Mathematics, 12.12.2021 16:40


Physics, 12.12.2021 16:50

English, 12.12.2021 16:50

Mathematics, 12.12.2021 16:50


Mathematics, 12.12.2021 16:50

Mathematics, 12.12.2021 16:50

World Languages, 12.12.2021 16:50

Social Studies, 12.12.2021 16:50

History, 12.12.2021 16:50

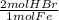 = 0.115 mol HBr
= 0.115 mol HBr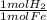 = 0.0573 mol H₂
= 0.0573 mol H₂

