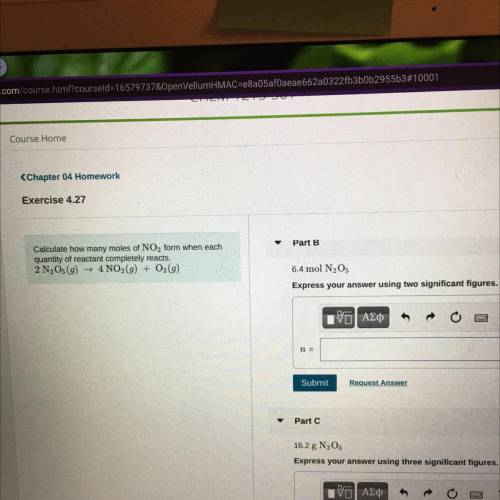
Calculate how many moles of NO2 form when each
quantity of reactant completely reacts.
2 N2O5...

Calculate how many moles of NO2 form when each
quantity of reactant completely reacts.
2 N2O5(9) + 4NO2(g) + O2(9)
Part B
6.4 mol N205
Express your answer using two significant figures.
VALO
n =
Submit
Request Answer
Part C
16.2 g N205
Express your answer using three significant figurer

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 12:00
Asubstance that will change shape to fit its container but has a definite volume is in a phase of matter
Answers: 1

Chemistry, 23.06.2019 12:00
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1
You know the right answer?
Questions

Mathematics, 05.01.2021 19:50

Chemistry, 05.01.2021 19:50

Mathematics, 05.01.2021 19:50


Mathematics, 05.01.2021 19:50





Biology, 05.01.2021 19:50

Physics, 05.01.2021 19:50

Mathematics, 05.01.2021 19:50

Biology, 05.01.2021 19:50

Mathematics, 05.01.2021 19:50






Health, 05.01.2021 19:50



