Part E
In part E, you'll use your answers from parts B, C, and D to find the
average density...

Chemistry, 13.03.2021 02:40 hickslily9
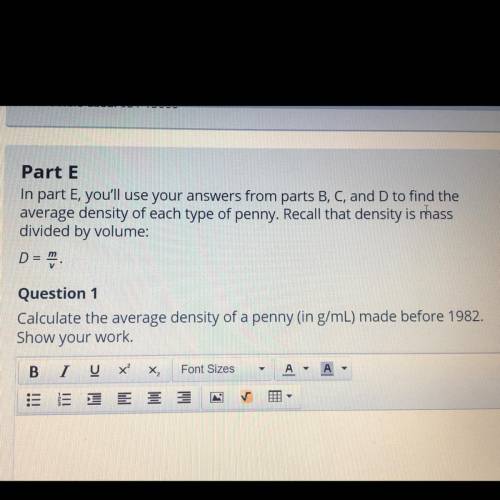
Part E
In part E, you'll use your answers from parts B, C, and D to find the
average density of each type of penny. Recall that density is mass
divided by volume:
D =
Question 1
Calculate the average density of a penny (in g/mL) made before 1982.
Show your work.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Questions


Computers and Technology, 15.10.2019 19:20


History, 15.10.2019 19:20


















