Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
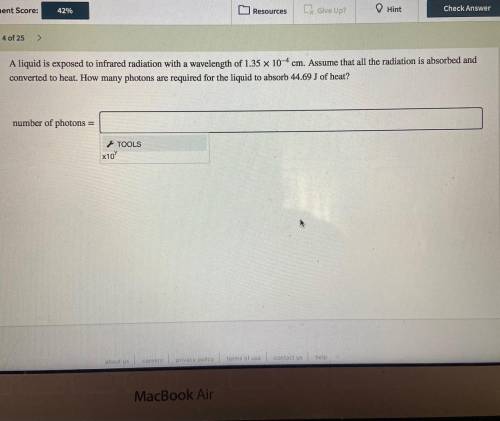
A liquid is exposed to infrared radiation with a wavelength of 1.35×10−4 cm.
Assume that all the ra...
Questions

Mathematics, 26.08.2019 09:50

History, 26.08.2019 09:50

Social Studies, 26.08.2019 09:50

Mathematics, 26.08.2019 09:50




Mathematics, 26.08.2019 09:50

Mathematics, 26.08.2019 09:50

Biology, 26.08.2019 09:50



Social Studies, 26.08.2019 09:50



Mathematics, 26.08.2019 09:50


Mathematics, 26.08.2019 09:50

Social Studies, 26.08.2019 09:50