
Chemistry, 12.03.2021 23:10 samafeggins2
Sulfur dioxide is a common preservative in wine; it prevents oxidation and bacterial growth. When SO2 is added to wine, it reacts with water to form an equilibrium system with the bisulfite ion:
SO2(aq) + H2O(l) ↔ H+(aq) + HSO3-(aq)
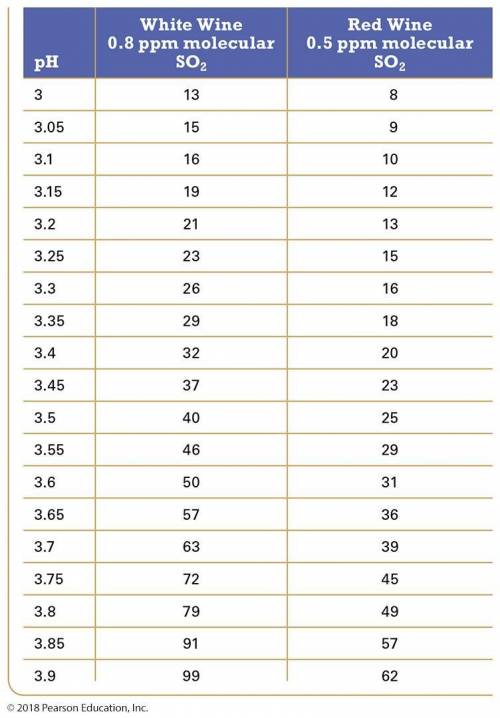
In this equilibrium system, SO2, is called "molecular SO2"; in its HSO3- form it is called "free SO2". Only molecular SO2 acts like a preservative. The amount of molecular SO2 in the equilibrium system is highly pH dependent. The lower the pH, the more the equilibrium shifts to the left and the greater the amount of free SO2 is present. (it favors the reactants). The recommended amount of free SO2 is 0.8 ppm for white wine and 0.5 ppm for red wine. The table below shows the amount of free SO2 required to obtain the correct amount of molecular SO2 as a function of pH for both red and white wine. For dilute solutions such as these, 1 ppm = 1 mg/L. Using the table values, answer the following questions.
[attachment]
Table: Amount of free SO2 required to maintain correct amounts of molecular SO2 in white and red wine.
Q#1: A 225 L barrel of white wine has an initial free SO2 concentration of 22 ppm and a pH of 3.70. How much SO2 (in grams) should be added to the barrel to result in the required molecular SO2 level?
Q#2: A 225 L barrel of red wine has an initial free SO2 concentration of 11 ppm and a pH of 3.80. How much SO2 (in grams) should be added to the barrel to result in the required molecular SO2 level?
Q#3: Gaseous SO2 is highly toxic and can be difficult to handle, so winemakers often use potassium metabisulfite (K2S2O5), also known as KMBS, as a source of SO2 in wine. When KMBS is added to wine, the metabisulfite ion (S2O52-) reacts with water to form the bisulfite ion. Write the balanced chemical equation for the reaction that occurs when the metabisulfite ions reacts with water.
Q#4: Determine the percent by mass of SO2 in KMBS.
Q#5: How much KMBS must a winemaker add to the barrels of wine in problems 1 & 2 to achieve the required amount of molecular SO2?
* I need help MOST on Q#5

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
Sulfur dioxide is a common preservative in wine; it prevents oxidation and bacterial growth. When SO...
Questions

Biology, 22.10.2020 06:01

Spanish, 22.10.2020 06:01






Biology, 22.10.2020 06:01

Social Studies, 22.10.2020 06:01



Social Studies, 22.10.2020 06:01

Social Studies, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01




Mathematics, 22.10.2020 06:01




