Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
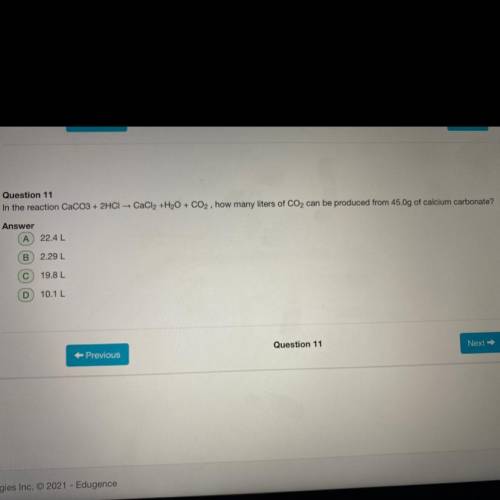
In the reaction CaCO3+2HCl CaCl 2 +H 2 O+CO 2 how many liters of CO 2 can be produced from 45.0g of...
Questions

Biology, 09.07.2019 20:20


Mathematics, 09.07.2019 20:20

Chemistry, 09.07.2019 20:20


History, 09.07.2019 20:20

Mathematics, 09.07.2019 20:20

Chemistry, 09.07.2019 20:20



Mathematics, 09.07.2019 20:20

English, 09.07.2019 20:20

English, 09.07.2019 20:20

English, 09.07.2019 20:20





World Languages, 09.07.2019 20:20