
Chemistry, 12.03.2021 18:20 savannahvargas512
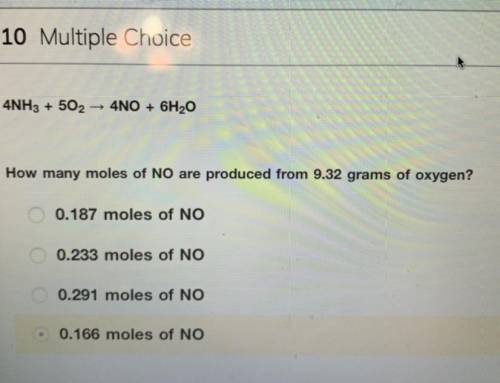
4NH3 + 5O2 > 4NO + 6H2O
How many moles of NO are produced from 9.32 grams of oxygen?
the answer is not 0.166 moles i tried and did not get credit for.

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
4NH3 + 5O2 > 4NO + 6H2O
How many moles of NO are produced from 9.32 grams of oxygen?
Questions

English, 21.01.2020 12:31

Chemistry, 21.01.2020 12:31

Mathematics, 21.01.2020 12:31



English, 21.01.2020 12:31





Health, 21.01.2020 12:31



Business, 21.01.2020 12:31

English, 21.01.2020 12:31

Physics, 21.01.2020 12:31


Spanish, 21.01.2020 12:31




