
Chemistry, 12.03.2021 16:30 pandasarecute53
Match the molecular formula to its empirical formula
1. C5H5
2.C11H22
3.C12H18O3
4.C6H12O2
my options are
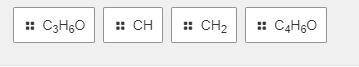

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

You know the right answer?
Match the molecular formula to its empirical formula
1. C5H5
2.C11H22
3.C12H1...
2.C11H22
3.C12H1...
Questions


History, 02.02.2021 01:50



Mathematics, 02.02.2021 01:50

Advanced Placement (AP), 02.02.2021 01:50

English, 02.02.2021 01:50

Mathematics, 02.02.2021 01:50

Mathematics, 02.02.2021 01:50

Geography, 02.02.2021 01:50

Chemistry, 02.02.2021 01:50





Social Studies, 02.02.2021 01:50


Mathematics, 02.02.2021 01:50

Mathematics, 02.02.2021 01:50

Mathematics, 02.02.2021 01:50



