
The nitrite ion is involved in the biochemical nitrogen cycle. You can determine the nitrite ion content of a sample using spectrophotometry by first using several reactions to form a colored compound from the ion. The following data were collected.
Nitrite Ion concentration (M) Absorbance of solution at 550 nm
2.00 x10^-6 M 0.065
6.00 x10^-6 M 0.205
10.00 x10^-6 0.338
14.00 x10^-6 0.474
18.00 x10^-6 0.598
Unknown 0.402
Required:
a. Construct a calibration plot, and determine the slope and intercept.
b. What is the nitrite ion concentration in the unknown solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 07:30
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m.a. calculate the kc value for the a-protein binding reaction.b. calculate the kc value for the b-protein binding reaction.c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1
You know the right answer?
The nitrite ion is involved in the biochemical nitrogen cycle. You can determine the nitrite ion con...
Questions




Business, 04.03.2022 19:50



Mathematics, 04.03.2022 19:50


Chemistry, 04.03.2022 19:50



English, 04.03.2022 19:50


Mathematics, 04.03.2022 19:50

Chemistry, 04.03.2022 20:00

Mathematics, 04.03.2022 20:00


Mathematics, 04.03.2022 20:00

Mathematics, 04.03.2022 20:00

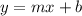 from of the line even for the x variable and also get on
from of the line even for the x variable and also get on 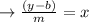
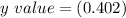 and the un-concentration is also in place
and the un-concentration is also in place


