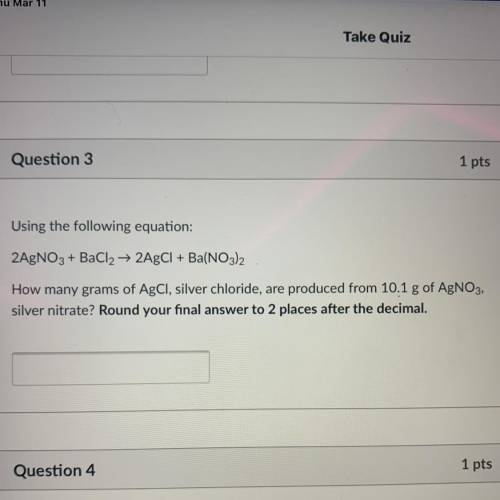
Using the following equation:
2AgNO3 + BaCl2 + 2AgCl + Ba(NO3)2
How many grams of AgCl, silve...


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Questions








English, 18.10.2019 23:30








Mathematics, 18.10.2019 23:30