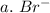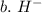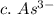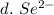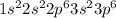a. fe

Chemistry, 02.02.2020 21:47 jazzycintron14
1.* write electron configurations for the 2 + 2 plus cations of these elements.
a. fe
b. co
c. ni
2.write electron configurations for the 3 + 3 plus cations of these elements.
a. chromium
b. manganese
c. iron
3. write the symbol for the ion formed when each element gains electrons and attains a noble-gas electron configuration.
a. br
b. h
c. as
d. se
4.* write electron configurations for the following atoms and ions, and comment on the result.
ar
cl − cap cl to the minus
s 2 − cap s super 2 minus end super
p 3 −

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

You know the right answer?
1.* write electron configurations for the 2 + 2 plus cations of these elements.
a. fe
a. fe
Questions


Physics, 21.06.2021 16:20














Mathematics, 21.06.2021 16:20

Social Studies, 21.06.2021 16:20



![a.\ Fe^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s 3d^{5} \ or \ [Ar]4s 3d^{5}](/tpl/images/0494/0749/a44c8.png)
![b.\ Co^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{5} \ or \ [Ar]4s^{2} 3d^{5}](/tpl/images/0494/0749/d425b.png)
![c.\ Ni^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{6} \ or \ [Ar]4s^{2} 3d^{6}](/tpl/images/0494/0749/26975.png)
![a.\ Cr^{3+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d \ or \ [Ar]4s^{2} 3d](/tpl/images/0494/0749/4de3e.png)
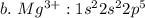
![c.\ Fe^{3+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{3} \ or \ [Ar]4s^{2} 3d^{3}](/tpl/images/0494/0749/51e46.png)