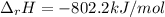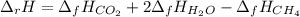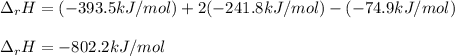Methane is the major component of natural gas. The standard molar enthalpy of formation of methane CH4 is -74.9 kJ/mol. For the balanced reaction equation of methane use:
CH4 + 2O2 --> CO2 + 2H2O.
All the chemicals in this reaction are gases. What is the enthalpy change of this reaction in kJ/mol?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2


You know the right answer?
Methane is the major component of natural gas. The standard molar enthalpy of formation of methane C...
Questions


Mathematics, 17.02.2021 23:20


English, 17.02.2021 23:20


Mathematics, 17.02.2021 23:20

Physics, 17.02.2021 23:20

Mathematics, 17.02.2021 23:20


Mathematics, 17.02.2021 23:20


Mathematics, 17.02.2021 23:20

Chemistry, 17.02.2021 23:20

Chemistry, 17.02.2021 23:20

English, 17.02.2021 23:20


Health, 17.02.2021 23:20

Health, 17.02.2021 23:20