
Chemistry, 11.03.2021 05:50 ilovemusicandreading
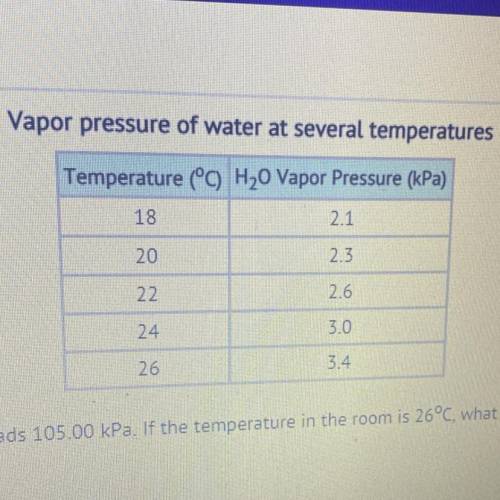
The barometer at an indoor pool reads 105.00 kPa. If the temperature in the room is 26°C, what is the partial pressure of the dry
air??
A
30.88 kPa
B)
101.60 kPa
108.40 kPa
D)
357.00 kPa

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
The barometer at an indoor pool reads 105.00 kPa. If the temperature in the room is 26°C, what is th...
Questions


Mathematics, 30.06.2020 09:01

Mathematics, 30.06.2020 09:01

Biology, 30.06.2020 09:01

Mathematics, 30.06.2020 09:01


Mathematics, 30.06.2020 09:01



Mathematics, 30.06.2020 09:01

Mathematics, 30.06.2020 09:01

English, 30.06.2020 09:01

Mathematics, 30.06.2020 09:01



Mathematics, 30.06.2020 09:01



English, 30.06.2020 09:01




