
Chemistry, 11.03.2021 05:10 gshreya2005
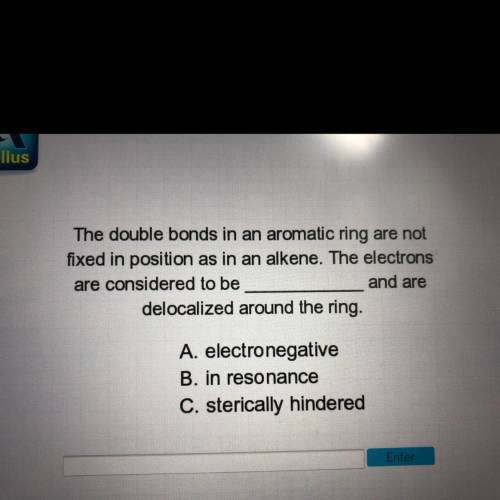
The double bonds in an aromatic ring are not fixed in position as in an alkene. The electrons are considered to be ___ and are delocalized around the ring.
A. Electronegative
B. In resonance
C. sterically hindered

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
The double bonds in an aromatic ring are not fixed in position as in an alkene. The electrons are co...
Questions

Social Studies, 17.09.2019 19:50

Biology, 17.09.2019 19:50

Mathematics, 17.09.2019 19:50

English, 17.09.2019 19:50



Social Studies, 17.09.2019 19:50

English, 17.09.2019 19:50


Chemistry, 17.09.2019 19:50

Mathematics, 17.09.2019 19:50



Health, 17.09.2019 19:50


Mathematics, 17.09.2019 19:50

History, 17.09.2019 19:50

Mathematics, 17.09.2019 19:50


Social Studies, 17.09.2019 19:50



