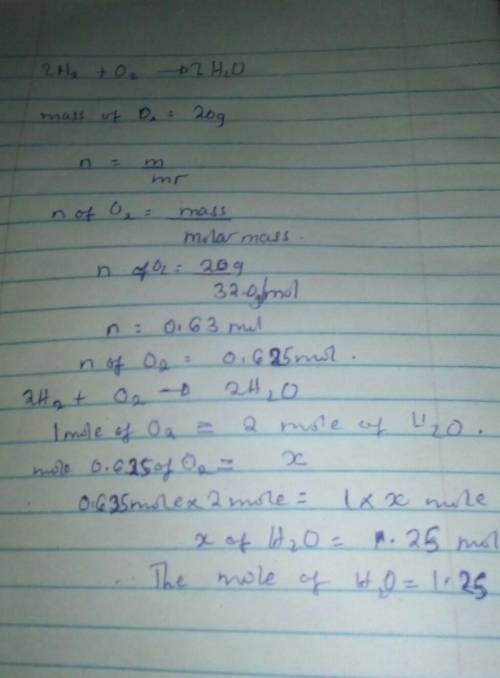Chemistry, 10.03.2021 23:00 trinidymwilga
Question 8 (1 point) 2H₂ + O₂ O2 --> 2 H20 Molar mass of H2 = 2.0 O2 =32.0 H2O=18.0 If you start with 20 grams of Oz, how many moles of H2O can be made? 1.25 mol 320 mol 1280 mol 0.31 mol Question 9 (1 point)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Question 8 (1 point) 2H₂ + O₂ O2 --> 2 H20 Molar mass of H2 = 2.0 O2 =32.0 H2O=18.0 If you start...
Questions


Social Studies, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

English, 19.11.2020 23:10


Mathematics, 19.11.2020 23:10




History, 19.11.2020 23:10

Spanish, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

English, 19.11.2020 23:10



Mathematics, 19.11.2020 23:10

World Languages, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

Chemistry, 19.11.2020 23:10