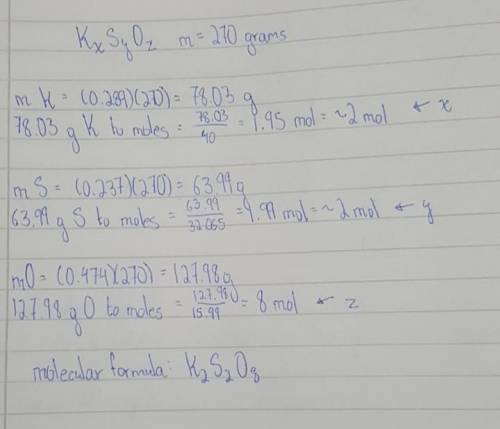Chemistry, 10.03.2021 20:50 ashgold324
An ionic compound is found to contain 28.9% potassium, 23.7% sulphur and 47.4% oxygen. The relative formula mass of the compound is 270. What is the formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
An ionic compound is found to contain 28.9% potassium, 23.7% sulphur and 47.4% oxygen.
The relative...
Questions



English, 07.11.2020 23:10



History, 07.11.2020 23:10


Mathematics, 07.11.2020 23:10

Mathematics, 07.11.2020 23:10

Law, 07.11.2020 23:10

Health, 07.11.2020 23:10




Mathematics, 07.11.2020 23:10





History, 07.11.2020 23:10