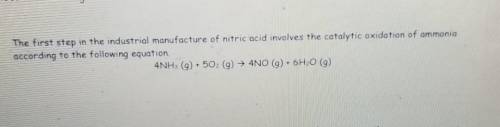How Many moles of oxygen(02) are needed to produce 4.6 g of nitrogen monoxide (NO)?
3.36 mol
...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Questions

Mathematics, 01.09.2021 05:20

English, 01.09.2021 05:20

English, 01.09.2021 05:20



Mathematics, 01.09.2021 05:20

Mathematics, 01.09.2021 05:20


Biology, 01.09.2021 05:20

Mathematics, 01.09.2021 05:20


History, 01.09.2021 05:20

Mathematics, 01.09.2021 05:20


Social Studies, 01.09.2021 05:20

Mathematics, 01.09.2021 05:20



Mathematics, 01.09.2021 05:20

Mathematics, 01.09.2021 05:20