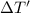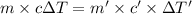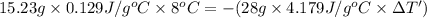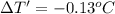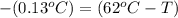Chemistry, 03.11.2019 17:31 lekaje2375
Apiece of gold with a mass of 15.23 g and an initial temperature of 54 °c was dropped into a calorimeter containing 28 g of water. the final temperature of the metal and water in the calorimeter was 62°c. what was the initial temperature of the water?
31.03 °c
62.13 °c
81.03°c
92.13°c

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
Apiece of gold with a mass of 15.23 g and an initial temperature of 54 °c was dropped into a calorim...
Questions

History, 23.11.2019 12:31


Mathematics, 23.11.2019 12:31


Biology, 23.11.2019 12:31

Mathematics, 23.11.2019 12:31


History, 23.11.2019 12:31

Mathematics, 23.11.2019 12:31

Mathematics, 23.11.2019 12:31

English, 23.11.2019 12:31

Health, 23.11.2019 12:31

Social Studies, 23.11.2019 12:31


Mathematics, 23.11.2019 12:31

Mathematics, 23.11.2019 12:31

Biology, 23.11.2019 12:31


History, 23.11.2019 12:31

Mathematics, 23.11.2019 12:31

 =62°C-54°C=8°C
=62°C-54°C=8°C