
Chemistry, 10.03.2021 18:40 esdancer3494
HELP ME PLSS
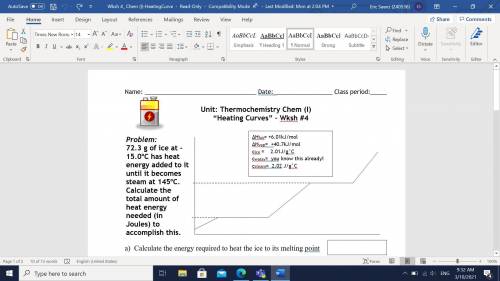
72.3 g of ice at -15.0oC has heat energy added to it until it becomes steam at 145oC. Calculate the total amount of heat energy needed (in Joules) to accomplish this.
A) Calculate the energy required to heat the ice to its melting point?
B) Calculate the energy required to melt the ice?
C) Calculate the energy required to heat the water to its boiling point?
D) Calculate the energy required to vaporize the water?
E) Calculate the energy required to heat the steam to 145oC?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1


Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
HELP ME PLSS
72.3 g of ice at -15.0oC has heat energy added to it until it becomes steam at 145oC....
Questions

Mathematics, 22.03.2021 01:20

Mathematics, 22.03.2021 01:20


Mathematics, 22.03.2021 01:20

Mathematics, 22.03.2021 01:20

Mathematics, 22.03.2021 01:20






Mathematics, 22.03.2021 01:20

Advanced Placement (AP), 22.03.2021 01:20



Spanish, 22.03.2021 01:20


Chemistry, 22.03.2021 01:20

Mathematics, 22.03.2021 01:20

Biology, 22.03.2021 01:20



