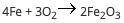Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1


Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
A student reacts 13 moles of iron with 21 moles of oxygen according to the following equation: (pict...
Questions

Physics, 08.12.2021 18:10

Biology, 08.12.2021 18:10


Mathematics, 08.12.2021 18:10


Mathematics, 08.12.2021 18:10


Mathematics, 08.12.2021 18:10


English, 08.12.2021 18:10




Social Studies, 08.12.2021 18:10

Mathematics, 08.12.2021 18:10

Mathematics, 08.12.2021 18:10

Mathematics, 08.12.2021 18:10

Mathematics, 08.12.2021 18:10

Chemistry, 08.12.2021 18:10

Mathematics, 08.12.2021 18:10