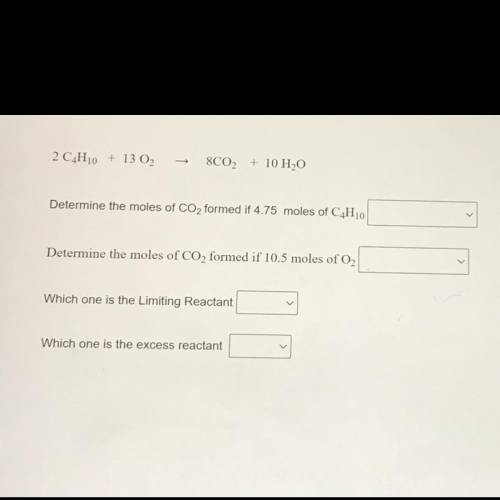
2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
...

Chemistry, 10.03.2021 04:00 vrentadrienneoqug1a
2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
<
Determine the moles of CO2 formed if 10.5 moles of O2
Which one is the Limiting Reactant
Which one is the excess reactant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
Questions

History, 22.06.2019 18:30





Mathematics, 22.06.2019 18:30

English, 22.06.2019 18:30

Social Studies, 22.06.2019 18:30

Mathematics, 22.06.2019 18:30

Chemistry, 22.06.2019 18:30


Biology, 22.06.2019 18:30

Social Studies, 22.06.2019 18:30


Mathematics, 22.06.2019 18:30

Mathematics, 22.06.2019 18:30

Mathematics, 22.06.2019 18:30

Mathematics, 22.06.2019 18:30


Health, 22.06.2019 18:30



