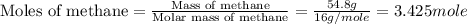Chemistry, 13.10.2019 05:01 averyeverdeen01
The molar heat of vaporization for methane, ch4, is 8.53 kj/mol. how much energy is absorbed when 54.8 g of methane vaporizes at its boiling point?
6.42 kj
29.1 kj
137 kj
467 kj

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
You know the right answer?
The molar heat of vaporization for methane, ch4, is 8.53 kj/mol. how much energy is absorbed when 54...
Questions

Mathematics, 01.04.2021 01:40

Mathematics, 01.04.2021 01:40

Mathematics, 01.04.2021 01:40

English, 01.04.2021 01:40


Mathematics, 01.04.2021 01:40

English, 01.04.2021 01:40

English, 01.04.2021 01:40

History, 01.04.2021 01:40

Mathematics, 01.04.2021 01:40

Mathematics, 01.04.2021 01:40


Mathematics, 01.04.2021 01:40

Mathematics, 01.04.2021 01:40

Social Studies, 01.04.2021 01:40


Advanced Placement (AP), 01.04.2021 01:40

Social Studies, 01.04.2021 01:40

Mathematics, 01.04.2021 01:40

Mathematics, 01.04.2021 01:40