
Chemistry, 09.03.2021 22:00 lilblackbird4
In the unbalanced equation shown below, how much iron was present before the reaction if there was 20 g of oxygen and 67 g of iron oxide produced?
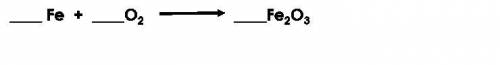

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
In the unbalanced equation shown below, how much iron was present before the reaction if there was 2...
Questions


Mathematics, 07.11.2019 13:31

Mathematics, 07.11.2019 13:31

Mathematics, 07.11.2019 13:31


Mathematics, 07.11.2019 13:31

Social Studies, 07.11.2019 13:31

Mathematics, 07.11.2019 13:31


Biology, 07.11.2019 13:31




English, 07.11.2019 13:31


History, 07.11.2019 13:31

Mathematics, 07.11.2019 13:31

History, 07.11.2019 13:31

Mathematics, 07.11.2019 13:31

Mathematics, 07.11.2019 13:31



