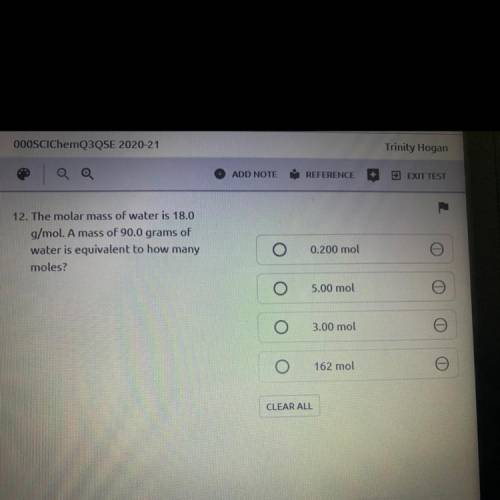
12. The molar mass of water is 18.0
g/mol. A mass of 90.0 grams of
water is equivalent to how...

Chemistry, 09.03.2021 21:40 jakhunter354
12. The molar mass of water is 18.0
g/mol. A mass of 90.0 grams of
water is equivalent to how many
moles?
HELP ASAP!!!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Questions



Mathematics, 27.05.2021 18:20

Chemistry, 27.05.2021 18:20


Health, 27.05.2021 18:20


History, 27.05.2021 18:20


Mathematics, 27.05.2021 18:20

Mathematics, 27.05.2021 18:20




Mathematics, 27.05.2021 18:20

Mathematics, 27.05.2021 18:20

Mathematics, 27.05.2021 18:20

Advanced Placement (AP), 27.05.2021 18:20

Mathematics, 27.05.2021 18:20

Mathematics, 27.05.2021 18:20



