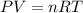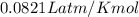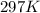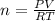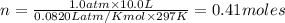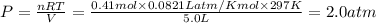Chemistry, 09.03.2021 20:10 2020davidhines
A sample of nitrogen (N2) gas in a 10.0L container has a
pressure of 1.0 atm at 297 K. Assuming ideal gas behavior,
what will the pressure be if the same amount of nitrogen
gas is put into a 5.0L container at 297 K?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

You know the right answer?
A sample of nitrogen (N2) gas in a 10.0L container has a
pressure of 1.0 atm at 297 K. Assuming ide...
Questions


History, 04.09.2020 22:01

Biology, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01

History, 04.09.2020 22:01

Biology, 04.09.2020 22:01

Geography, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01




Mathematics, 04.09.2020 22:01





Geography, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01