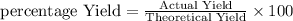Question 5
1 pts
What is the correct interpretation of an 85% yield for a given reactio...

Chemistry, 09.03.2021 04:10 lrich20200
Question 5
1 pts
What is the correct interpretation of an 85% yield for a given reaction?
85% of the anticipated amount of products were produced (relative to the theoretical yield)
O 15% of the reactants were used up
O 85% of the reactants were used up
15% of the anticipated amount of products were produced (relative to the theoretical yield)
Question 6
1 pts
True or false: The limiting reactant is the reactant that is used up after the reaction is complete.
O True
False

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
You know the right answer?
Questions


Mathematics, 17.04.2020 02:54


History, 17.04.2020 02:54



Mathematics, 17.04.2020 02:54






Mathematics, 17.04.2020 02:54